Of course, you can also reach us via e-mail or our contact form. We will get in touch with you as soon as possible.
You can find this content here:
QM as Competitive Advantage | Acceptance of QMS | Standards & Regulations | QM Software from Babtec
Why do companies whose quality management is effective have a competitive advantage? Anyone who has ever asked themselves this question will have come across the following reasons:
In addition to quality management requirements, companies are also subject to requirements from areas such as environmental protection or occupational health and safety. So why not look directly beyond quality management?
An integrated management system (IMS) is a strategic approach that combines these requirements into one system. The result: a single system – with great potential.
The basis of management system standards is the Harmonized Structure (formerly High Level Structure). Different management system standards can be integrated more easily thanks to this uniform basic structure. You can find more information on this in our blog article on the topic of Harmonized Structure / High Level Structure.
The advantages of an integrated management system are obvious:
A QMS must not only be introduced in a company, it must also be lived. Only then will it be effective in the long term. In both cases, the QM software plays an important role: the available functions contribute to the introduction of the quality management system (QMS). It is also conducive to the acceptance of QMS, as it offers those involved transparency and scope for co-design.
If a QM system is to be introduced within a company, various aspects must be taken into account. Above all, the leadership and commitment of the company management is essential. The latter has a role model function. It is also necessary to plan resources in terms of people, time and budget in order not only to introduce the QM system but also to maintain it.
In addition, companies should define clear quality objectives and strategies in order to use these as the basis for the QM system. The analysis and documentation of existing processes can also be incorporated into this.
Document control is also very important, by means of which relevant documents and procedural instructions are properly managed, updated and controlled.
In order not only to introduce the QM system, but also to live it, employees need to be trained. They must understand the requirements of the system so that they can implement and live them. By means of internal audits, companies continuously put the QM system to the test in order to identify potential weaknesses.
QM software provides a remedy when it comes to introducing a QM system. It provides functionalities that benefit the above-mentioned challenges:
Document Control
QM software enables the central storage and management of documents, guidelines and procedures. This ensures that everyone involved always has access to the latest versions.
Automation of QM Processes
Automating quality processes and workflows makes it easier to carry out the processes defined in the QM system, such as inspections or failure mode and effect analyses.
Risk Management
QM software can support the identification, evaluation and monitoring of potential risks – and even generate warnings in the event of deviations or critical events.
Audit Management
QM software supports the planning, implementation and follow-up of internal and external audits. This makes it easier to ensure compliance with standards and regulations.
Training Management
The ability to manage training activities within the QM software also means that training requirements can be identified. In this way, companies ensure that employees have the necessary skills for their tasks.
Provision of Key Figures
QM software makes it easier to create dashboards and reports. This not only makes it easy to track the development of quality, but also to check in which areas there is a need for improvement. The analysis of quality data helps to identify patterns and make well-founded decisions.
Documentation
Quality processes that are mapped using QM software are automatically documented and can also be viewed and traced at a later date. This makes it easier to comply with regulations and provide corresponding evidence.
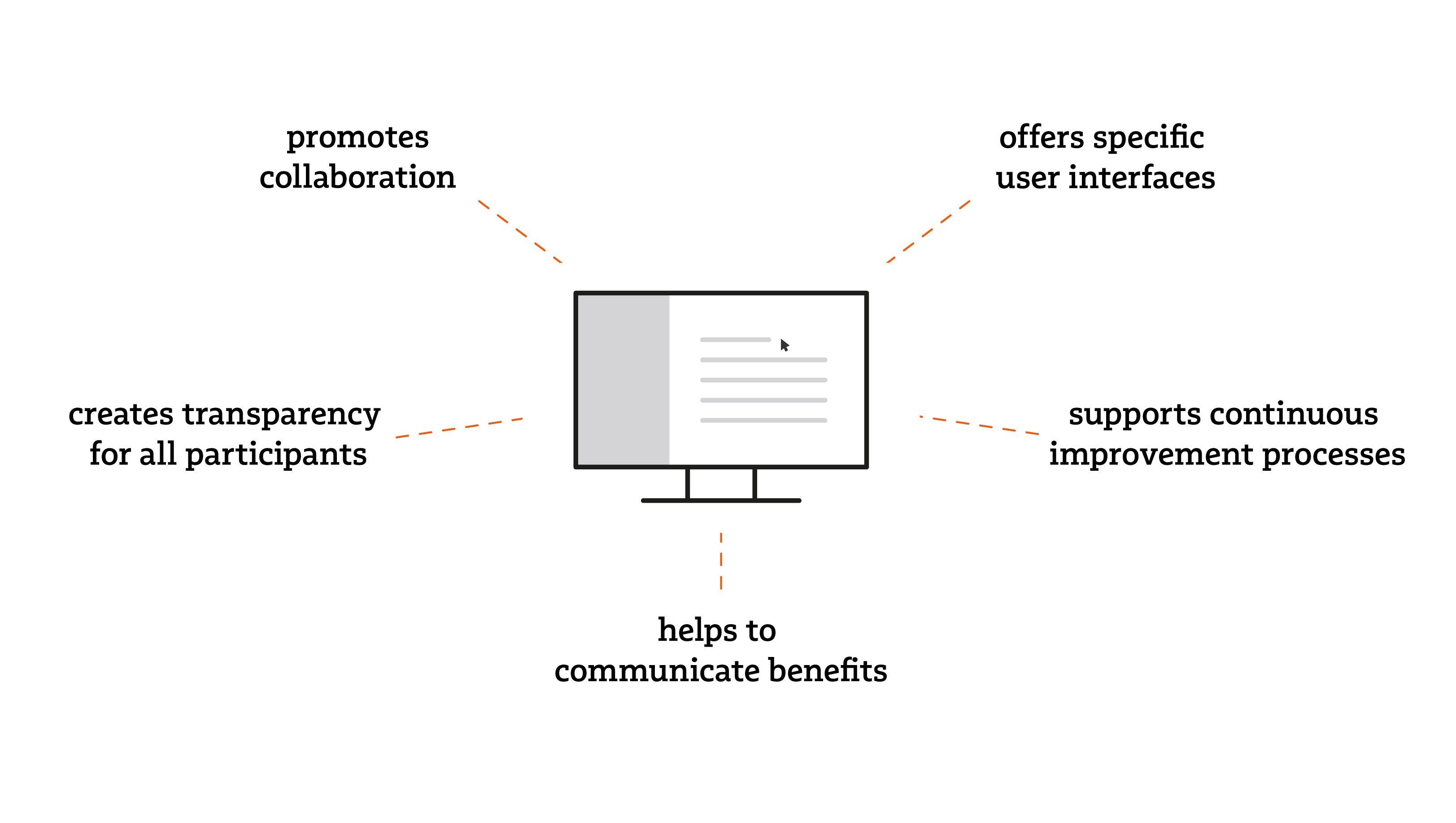
The use of QM software helps you to increase the acceptance of your quality management system (QMS).
By using QM software, a company can promote the acceptance of the QMS.
Whether standards and regulations are obligations or opportunities may vary from case to case. Depending on the industry, company and customer, various standards are certainly (unspoken) obligations. However, meeting additional requirements can also be an opportunity.
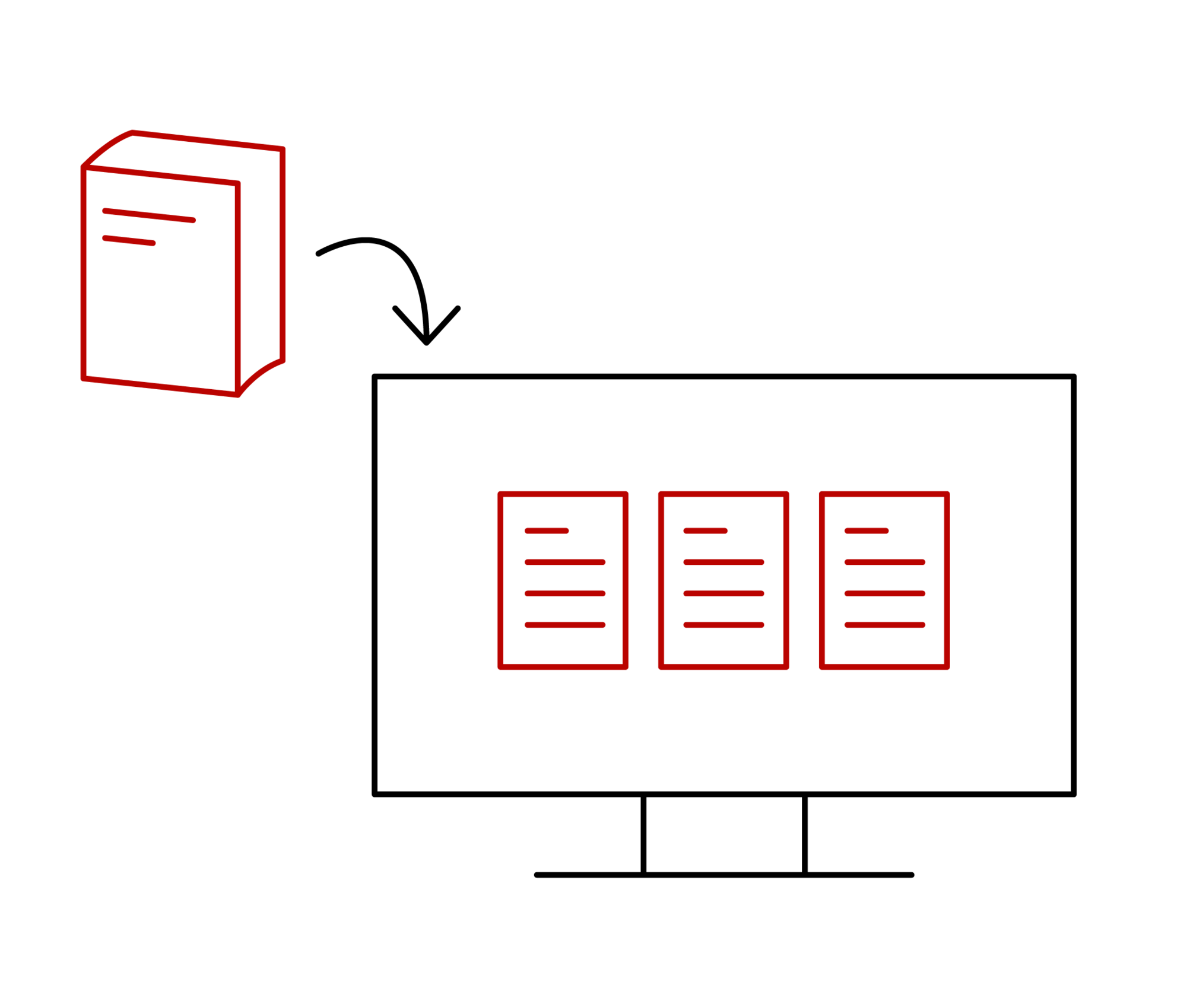
An electronic QM manual is basically the digital version of a quality management manual. It is used for the electronic storage of all relevant information and documentation relating to the quality management system.
An (electronic) QM manual is not or no longer explicitly prescribed in standards. Nevertheless, the need for effective document control and management is a relevant issue – and the most effective way is to use an electronic solution. This offers companies the following advantages in particular:
You want to achieve the best results for your product or service in terms of quality – we want to provide you with the best possible support. That's why we offer you the choice between two product worlds: BabtecQ supports comprehensive quality management with a high level of detail and the best process integration for your company. The cloud-based BabtecQube fulfills the desire for tried-and-tested, intuitive and immediately available standard tools.
for all those who have high demands on the level of detail of their quality control loop.
Features
Quality processes to be mapped
A total of 21 modules: APQP, Requirements Management, Audits, Task and Action Management, CAD Integration, CAPA, Checklists, Control Plan, Document Control, Initial Sampling, Production Inspection, FMEA, Maintenance, Supplier Assessment, Gage Management, Process Management, Qualification and Training Management, Complaints Management, Incoming and Outgoing Goods Inspection, Warranty Management, Quality Cockpit
Services
Costs
Depending on the required modules, interfaces and customizing needs;
you will receive an individual offer on request

for all those who have less high demands on the level of detail of their quality control loop.
Features
Quality processes to be mapped
A total of 5 services: Complaints & Deviations, Goods Inspections & Checklists, Equipment & Gages, Tasks & Actions, Analyses
Services
Costs
Basic account free of charge; premium subscription from €29 per month per user
 Product Worlds
Comprehensive quality management or smart solution in the cloud: The choice is yours
Product Worlds
Comprehensive quality management or smart solution in the cloud: The choice is yours
 Take responsibility for quality management
How your quality management gets a little better every day with Babtec
Take responsibility for quality management
How your quality management gets a little better every day with Babtec
 High Level Structure: The Basis of Management System Standards
In this BloQ article, we take a look at the High Level Structure and its benefits.
High Level Structure: The Basis of Management System Standards
In this BloQ article, we take a look at the High Level Structure and its benefits.